Projects
Elucidating the Architecture of Plant Gene Regulatory Networks
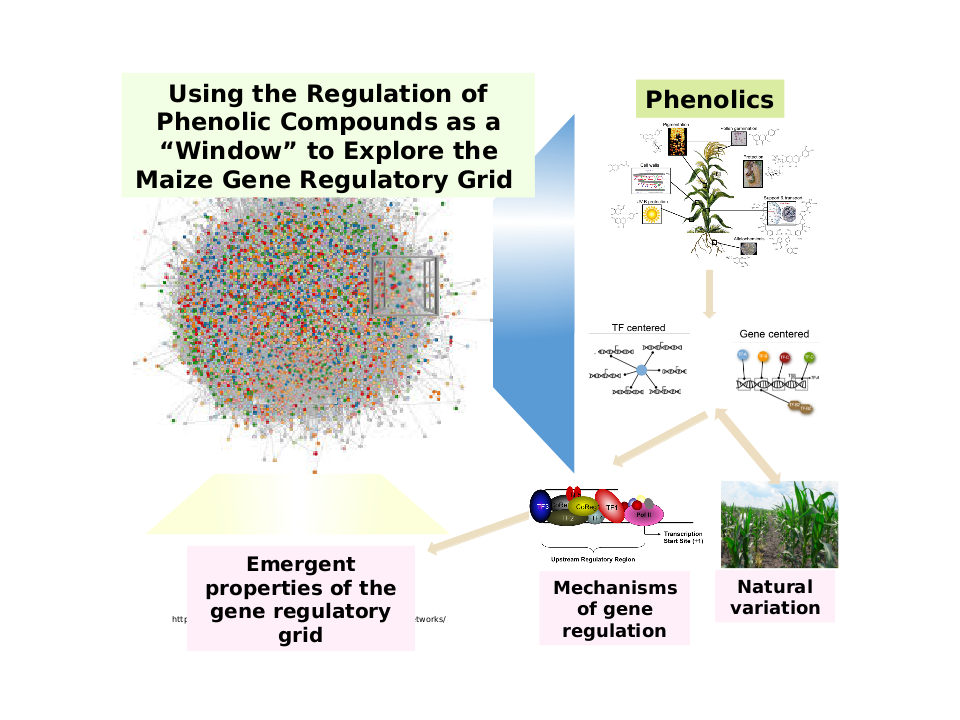
Figure 1. Using the Regulation of Phenolic Compounds as a Window for Exploration into the Maize Gene Regulatory Network. The integration of multiple omics data related to phenolic metabolism helps reveal the properties of the gene regulatory grid in maize. Focusing on all different types of phenomics, genomics, transcriptomics, and metabolomics data allow us to study the complex inter-relationships and how they function toward influencing phenotypes, such as phenolic compounds accumulation in maize population. With the rapid expansion within the omics field, consolidating multiple approaches are essential to provide new perspectives on how the gene regulatory grid/network makeup of maize.
Elucidating the architecture of gene regulatory grids and gene regulatory networks involves linking the cis-regulatory machinery (cistrome) with the trans-acting factors through the identification of protein-DNA interactions (PDIs). Our lab combines gene- and transcription factor (TF)-centered approaches to determine the biologically-relevant PDIs important for the temporal and spatial expression of all genes. Experiments are largely conducted in maize and Arabidopsis as well as projects in tomato and Camelina. In tomato, we are investigating the interaction of several TFs with target genes involved in early fruit development. In maize, we implemented DNA affinity purification sequencing (DAP-seq) and chromatin immunoprecipitation sequencing (ChIP-seq) to establish the genome-wide occupancies of 45 TFs involved in the control of the phenylpropanoid pathway. The cistrome hinges on where transcription initiation starts, thus, our lab has been conducting extensive cap analysis of gene expression (CAGE) to determine the location of transcription start sites (TSS) in two maize inbred lines, B73 and Mo17, under normal and stress conditions. Additionally, we aim to understand how TSS selection is controlled in cis/trans; thus, we have conducted CAGE sequencing and computational analysis on F1 hybrids to generate unparalleled information on how TSSs are determined.
Biosynthesis, Transport and Regulation of Flavonoids and Other Phenolic Compounds
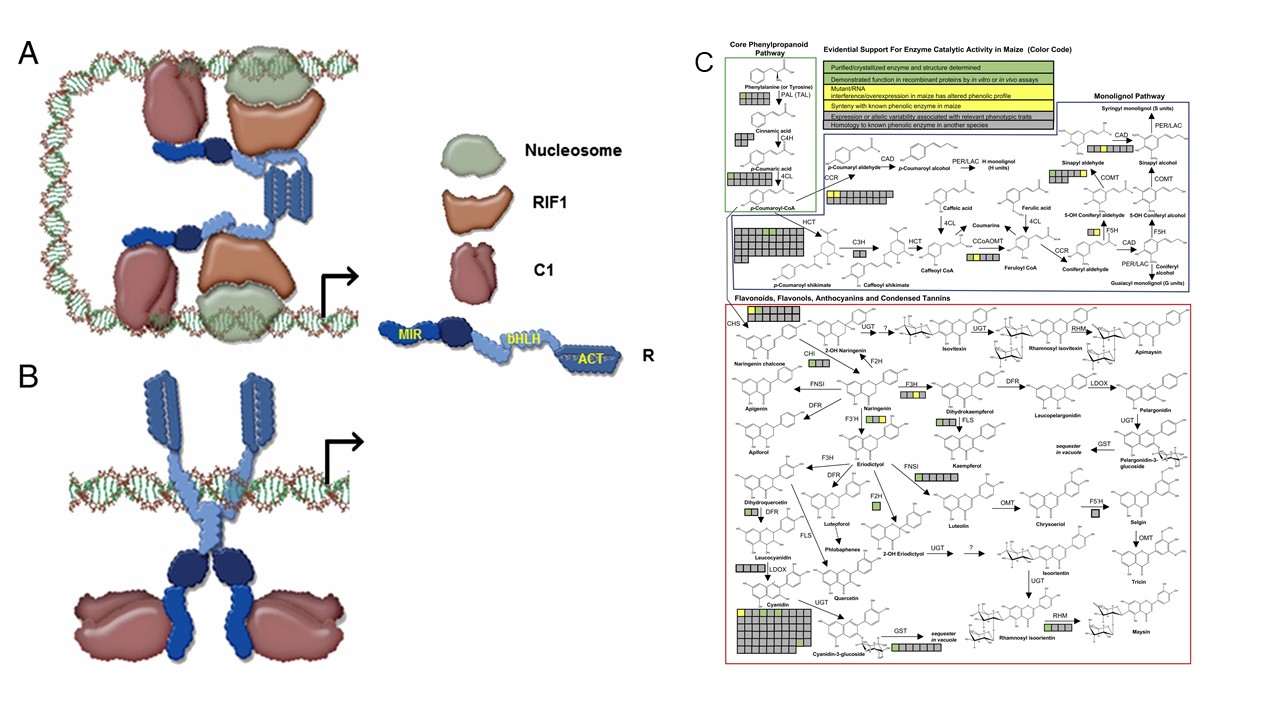
Figure 2. Biosynthesis, Transport and Regulation of Flavonoids and Other Phenolic Compounds. Proposed model for promoter switching by R (bHLH protein) to regulate anthocyanin accumulation in association with C1 (R2R3-MYB protein; 2A) and RIF1 (EMSY-related protein) in Maize (Kong et al 2012; 2B). Summary of evidence for Core Phenylpropanoid, Monolignol, and Flavonoid Pathways in Maize (Gomez-Cano et al 2020; 2C).
Phenolic compounds are important plant specialized metabolites that participate in biotic and abiotic interactions between plants and the environment. Especially, Flavonoid-pigments, including the anthocyanins and phlobaphenes, have provided powerful visual tools to understand fundamental biological concepts. Our lab is interested to investigate how the interaction between R2R3-MYB and basic helix-loop-helix (bHLH) transcription factors cooperate to regulate anthocyanin accumulation in maize and Arabidopsis; and how bHLH transcription factors with very similar DNA-binding domains display regulatory specificity, for example in the regulation of flavonoid pigments in maize aleurone, and in Arabidopsis epidermal cell differentiation (e.g., trichomes). Meanwhile, our lab is using anthocyanin compounds to better understand how specialized metabolites traffick within cells, an issue for the vast majority of thousands of specialized metabolites that characterizes land plants. Given the importance of phenylpropanoids and lignin compounds for agricultural biomass and biofuel potential, our lab is also focused on the identification of new genes involved in the biosynthesis and regulation of maize phenylpropanoids, using a combination of yeast and transient expression in Nicotiana benthamiana.
Engineering the Pathways for Insecticidal and Nutritional Flavones
Figure 3. Engineering the Pathways for Insecticidal and Nutritional Flavones. A transgenic tomato plant harboring the gene, ZmFNSI-1, driven by the pMKS1 trichome specific promoter, encodes a flavone synthase, which uses the flavonones naringenin and eriodyctiol to produce the flavones apigenin and luteolin, respectively (A). By expressing ZmFNSI-1 in a tissue specific manner, we aim to obtain flavones beneficial to human health and precursors (luteolin) in maysin biosynthesis, our main target to engineer in tomato. To engineer flavones and C-glycosylflavones involved in the maysin biosinthesis, all the enzymes are being expressed in a tissue specific manner using the pMKS1 promoter (B) and in constitutive manner using the promoter of the Chalcone synthase gene, pCHI1 (C).
One group of flavonoid compounds with human health importance and that also provide resistance to herbivores and other pathogens are flavones. In maize, C-glycosylflavones, in particular maysin, provide significant protection to the corn earworm (Helicoverpa zea) and related insects when they accumulate in the silks. We have elucidated the pathway for maysin biosynthesis, and we are currently engineering this pathway into other crops and vegetables that are susceptible to Helicoverpa zea. Using stable transformation in tomato and transient expression in Nicotiana benthamiana, we are expressing the necessary enzyme-coding genes to produce all the flavones and C-glycosyl flavones involved in maysin biosynthesis. Because glycosylated flavones are usually less health-beneficial to animals than the aglycones, we are also combining available maize mutants to enhance the accumulation of seed flavone aglycones, in particular apigenin and luteolin.
Biosynthesis and Regulation of Seed Oils in Emerging Oilseed Crops

As part of several collaborative projects, the lab is involved in elucidating the mechanisms by which seed oils are regulated in Camelina and Pennycress, and how this knowledge can be translated into strategies to enhance oilseed production in these plants with increasing agronomic potential. We are currently working on transforming candidate genes responsible for enhancing or regulating seed oil production in Camelina and Pennycress.  Transgenes altering oil accumulation allow us to increase fundamental knowledge of the not fully understood fatty acid biosynthetic pathways, as well as, enable targeted breeding to increase the yield of oil in the seed. In addition, we also plan to accomplish this goal with DNA affinity purification sequencing (DAP-seq) strategy, an in vitro TF centered technique, to identify potential TF-DNA interactions responsible for elevating oil levels.
Image courtesy of: CINTIA ARIAS of University of North Texas, Texas (UNT)
Having Issues with the website? Contact Oliver with any questions or concerns.
.png)