Current Research
My laboratory investigates microbial physiology and enzymology related to transition metals. We study the mechanisms of catalysis by metalloenzymes and characterize the biosynthesis of their protein metallocenters. We use an array of experimental techniques and approaches that includes gene cloning, site-directed mutagenesis, enzyme kinetics, biophysical spectroscopic methods, and protein structural methods.
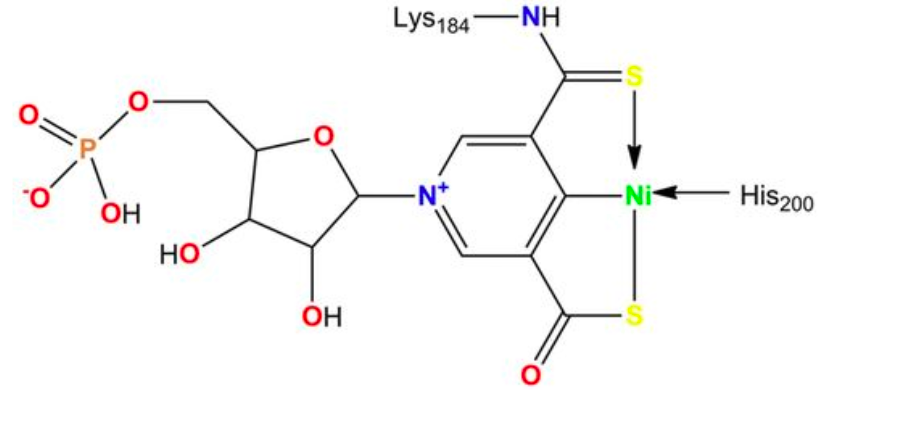
One major emphasis in my laboratory focuses on the characterization of racemases and epimerases that possess the nickel-pincer nucleotide (NPN) cofactor, which features a nickel-carbon bond. We discovered this cofactor covalently tethered to lactate racemase (Science 349:66-69, 2015) and have since shown it occurs in a large family of enzymes. Our studies also investigate how bacterial cells synthesize this niacin-derived complex from nicotinic acid adenine dinucleotide using three enzymes: the LarB carboxylase/hydrolase, the LarE sulfur insertase, and the LarC nickel insertase or cyclometallase.
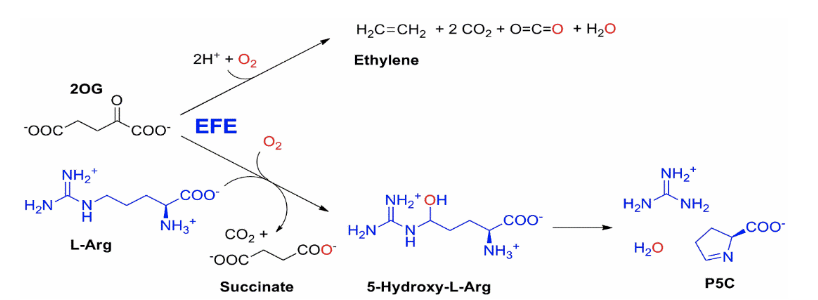
A second area of emphasis centers on several ferrous ion and 2-oxoglutarate (2OG) dependent hydroxylases (Trend Biochem. Sci. 32:517-532, 2018). TauD functions in the bacterial metabolism of sulfonated compounds and has become the paradigm of this enzyme family because of our spectroscopic, mutagenic, and crystallographic studies. Current work focuses on a bacterial or fungal ethylene-forming enzyme that exhibits two reactions: conversion of 2OG to ethylene plus three CO 2 /bicarbonate or transformation of L-arginine to guanidine plus pyrroline- 5-carboxylate coupled to 2OG decomposition to succinate plus CO2.
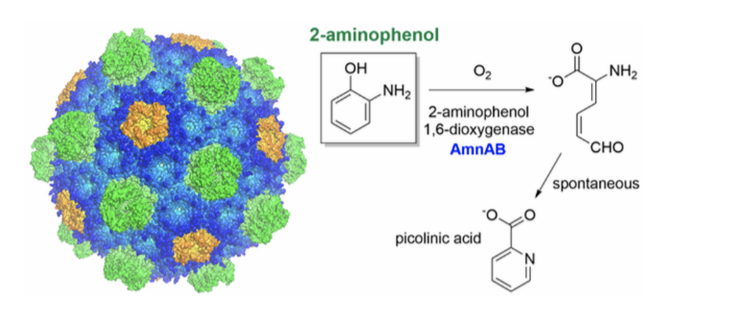
A third research area studies the effect of enzyme confinement within bacteria microcompartments. The enzyme of interest is an iron-dependent 2-aminophenol 1,6- dioxygenase.