Research
Our research falls into two broad categories – carbon metabolism of photosynthesis and isoprene emission.
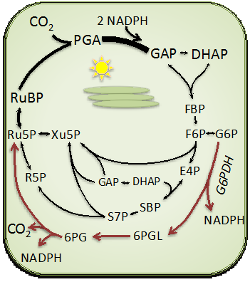
Carbon metabolism — Changes in the carbon dioxide concentration in the atmosphere will have profound effects on photosynthesis (it already has). We study the carbon inputs into, and outputs from, the Calvin Benson Cycle. Carbon input studies are mostly focused on diffusion resistances for carbon dioxide (Sharkey 2012; Weise et al. 2015; Sharkey 2016). A major project is understanding a hypothesized glucose-6-phosphate shunt. In essence, carbon fixed by rubisco is released in the oxidative branch of the pentose phosphate pathway. We hypothesize this helps keep the Calvin Benson Cycle intermediates available when light levels change rapidly (Sharkey & Weise 2016). We hypothesize this explains the observation that the Calvin Benson cycle intermediates do not fully label when labeled carbon dioxide is fed to leaves. Finally, we study how leaf photosynthesis is connected to plant growth. When the photosynthetic rate per leaf area is examined, it does not explain many differences in growth rates of plants. The amount of dry matter per area of a leaf is a much better of plant growth rate. Leaf mass per area is a measure of the investment of resources for a given amount of whole plant photosynthesis and also affects the respiration cost of making new photosynthetic leaf area (Sharkey 2015; Weraduwage et al. 2015; Weraduwage et al. 2016).
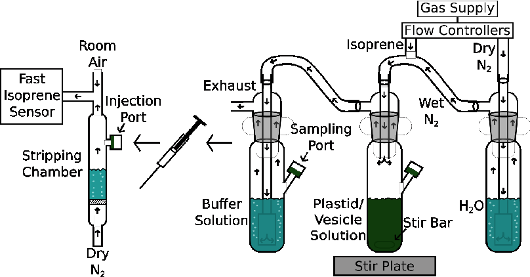
Isoprene — Isoprene emission from plants is greater that any other hydrocarbon input into the atmosphere. This isoprene affects atmospheric chemistry. In polluted atmospheres it can make ozone worse, in unpolluted atmospheres it can contribute to aerosols that make a blue haze often seen in mountainous areas (thus Blue Ridge Mountains in Southeastern US, the Blue Mountains in Australia). Despite the vast quantity of isoprene emitted by some plants, especially trees like oaks, poplars, and eucalypts, we still do not know why plants emit isoprene. There clearly is some protection against abiotic stress such as high temperature and ozone as a result of isoprene production in plants but the mechanism of this abiotic stress protection is unclear. Recently, we provided evidence that cast doubt on the two leading hypotheses, that isoprene was modifying membrane properties or was quenching reactive oxygen (Harvey et al. 2015). But not all was lost, we also saw gene expression changes (Harvey & Sharkey 2016) that were consistent with observations of colleagues using a different plant system (Behnke et al. 2010). Current questions are how does isoprene affect gene expression and how does the altered gene expression provide abiotic stress tolerance.
References
Behnke K. Kaiser A. Zimmer I. Brüggemann N. Janz D. Polle A., . . . Schnitzler J.-P. (2010) RNAi-mediated suppression of isoprene emission in poplar transiently impacts phenolic metabolism under high temperature and high light intensities: a transcriptomic and metabolomic analysis. Plant Molecular Biology 74, 61-75.
Harvey C.M., Li Z., Tjellström H., Blanchard G.J. & Sharkey T.D. (2015) Concentration of isoprene in artificial and thylakoid membranes. Journal of Bioenergetics and Biomembranes 47, 419-429.
Harvey C.M. & Sharkey T.D. (2016) Exogenous isoprene modulates gene expression in unstressed Arabidopsis thaliana plants. Plant Cell and Environment 39, 1251-1263.
Sharkey T.D. (2012) Mesophyll conductance: Constraint on carbon acquisition by C3 plants. Plant Cell and Environment 35, 1881-1883.
Sharkey T.D. (2015) Understanding carbon partitioning and its role in determining plant growth. Plant Cell and Environment 38, 1963-1964.
Sharkey T.D. (2016) What gas exchange data can tell us about photosynthesis. Plant, Cell & Environment 39, 1161-1163.
Sharkey T.D. & Weise S.E. (2016) The glucose 6-phosphate shunt around the Calvin-Benson Cycle. Journal of Experimental Botany 67, 4067-4077.
Weise S.E., Carr D.J., Bourke A.M., Hanson D.T. & Sharkey T.D. (2015) The arc mutants of Arabidopsis with fewer large chloroplasts have a lower mesophyll conductance. Photosynthesis Research 124, 117-126.
Weraduwage S.M., Chen J., Anozie F.C., Morales A., Weise S.E. & Sharkey T.D. (2015) The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Frontiers in Plant Science 6, 167.
Weraduwage S.M., Kim S.-J., Renna L., Anozie F.C., Sharkey T.D. & Brandizzi F. (2016) Pectin methylesterification impacts the relationship between photosynthesis and plant growth. Plant Physiol 171, 833-848.