Research: Project 2
 Thermophilic oxidoreductases as industrial catalysts. Dehydrogenases transfer an electron pair and a proton between a cofactor and a substrate.
These enzymes could be used to produce many industrial compounds, but their applications
remain limited to high-value products due to the high costs associated with cofactor
regeneration. In collaboration with Drs. R. Mark Worden and Scott Calabrese Barton
(both from MSU’s Department of Chemical Engineering and Materials Science), we have
been using Thermotoga maritima glycerol dehydrogenase (TmGlyDH), which produces dihydroxyacetone (DHA) from glycerol,
to demonstrate that dehydrogenase-catalyzed reactions can be driven by NADH recycling
at an electrode surface.
Thermophilic oxidoreductases as industrial catalysts. Dehydrogenases transfer an electron pair and a proton between a cofactor and a substrate.
These enzymes could be used to produce many industrial compounds, but their applications
remain limited to high-value products due to the high costs associated with cofactor
regeneration. In collaboration with Drs. R. Mark Worden and Scott Calabrese Barton
(both from MSU’s Department of Chemical Engineering and Materials Science), we have
been using Thermotoga maritima glycerol dehydrogenase (TmGlyDH), which produces dihydroxyacetone (DHA) from glycerol,
to demonstrate that dehydrogenase-catalyzed reactions can be driven by NADH recycling
at an electrode surface.
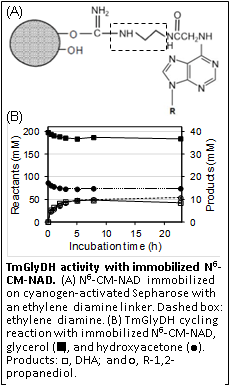
My laboratory characterized the general stability and kinetic properties of TmGlyDH to identify the best conditions for catalysis. We showed that TmGlyDH is active with the NAD analog N6-carboxymethyl-NAD+ immobilized on Sepharose through a diamino linker, suggesting that the NAD analog can be immobilized on an electrode to allow TmGlyDH activity in a system that reoxidizes the cofactor electrocatalytically.
DHA inactivates TmGlyDH at elevated temperatures by reacting with Arg and Lys residues. Work is in progress to identify the Arg and Lys residues that are modified by DHA. Substitutions that stabilize TmGlyDH in the presence of DHA will be identified and combined in the same enzyme to increase TmGlyDH resistance to DHA inactivation.