Research Spotlight: Dr. Yousi Fu
Dr. Yousi Fu is a postdoctoral research associate in the laboratory of Professor Rob Quinn, and first author of the group’s latest publication appearing in Nature Communication — “Balance between bile acid conjugation and hydrolysis activity can alter outcomes of gut inflammation.”
The College of Natural Science spoke with Dr. Fu to learn more about the team’s discoveries, and the latest breakthroughs achieved by Spartan biochemists.

The Quinn Lab accomplishes pioneering science in the context of microbial communities and their impact on health and disease. Can you speak a bit more about the research happening in the lab and the questions you pursue?
Our lab is dedicated to unraveling how microbial communities in the gut shape health and disease, with a particular focus on the role of bile acids—molecules critical for digestion, immune regulation, and overall gut homeostasis. In patients with inflammatory bowel disease (IBD), bile acid metabolism is often disrupted, which we think could be target for therapeutics and treatments.
However, the specific roles and impacts of bile acids, especially those conjugated bile acids, in IBD remain poorly understood. Our research seeks to clarify these mechanisms, asking questions like: How do conjugated bile acids influence inflammation in IBD? We aim to uncover how bile acid dysregulation contributes to disease and identify new therapeutic targets to restore gut health.
Our research spans from mapping microbial metabolism in inflammatory conditions like IBD to discovering novel microbial compounds with therapeutic potential. We leverage cutting-edge tools, including novel gene knockout mouse models, metabolomics, and metagenomics, to answer critical questions about how microbial activities shape gut homeostasis and contribute to disease, paving the way for innovative treatments. Oh, and we also study coral reefs and lung diseases too. There’s a lot going on in the Quinn lab!
What originally drew you to become interested in this area of research, and particularly the gut microbiome?
My fascination with microbiology began during my undergraduate studies, where I was captivated by bacterial fermentation engineering—how microbes could transform simple substrates into valuable products.
During my PhD, I encountered fecal microbiota transplantation (FMT), a treatment that uses human gut microbiome to restore health, especially for IBD. Learning about FMT opened my eyes to the gut microbiome’s profound influence on human health, revealing the potential to manipulate the gut microbiome to improve health. Bile acids are one of the most important signaling molecules in humans. I wanted to dive into discover how bacteria manipulate these molecules—and how that shapes inflammation—it sort of like solving a puzzle with real clinical implications.
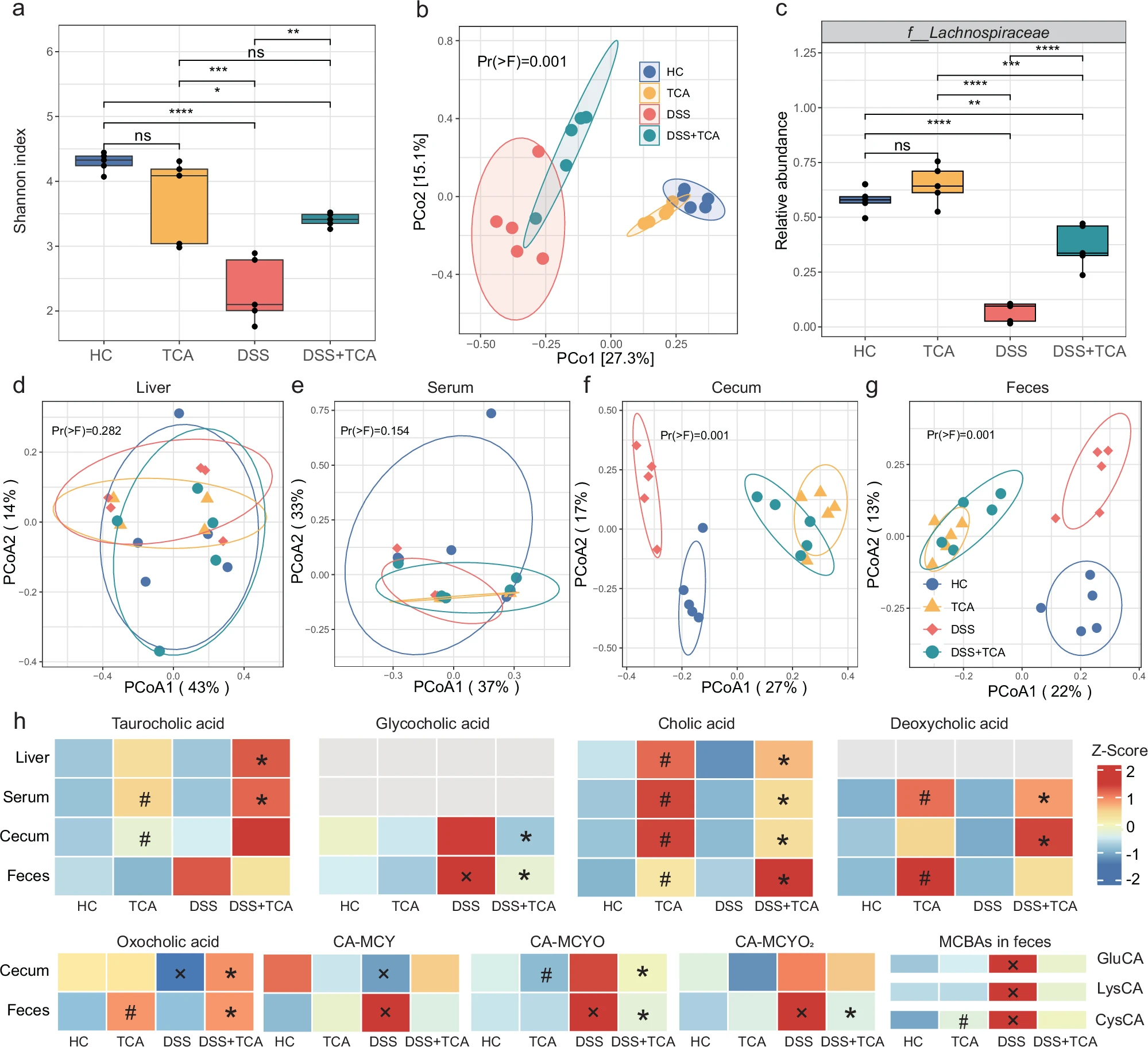
Your latest publication sheds light on the relationship between gut inflammation and bile acids—some of the most abundant and crucial molecules in the gut. What exactly did the team discover?
Our research explored how molecules in the gut, named bile acids, influence inflammation—a painful swelling that happens in conditions like inflammatory bowel disease (IBD). Bile acids are produced by the liver and then modified by gut bacteria, and we discovered that getting the right balance of these changes is crucial in IBD. In our mouse studies, we found that adding a specific bile acid, called taurocholate or TCA, calmed inflammation by reinforcing the gut’s natural protective barrier and boosting the growth of beneficial bacteria.
What's most exciting about these findings?
What excites us most is how this balance isn’t just about one molecule—it involves a dynamic interplay between the host and microbiome, opening up new ways to think about treating inflammation.
What’s most remarkable to me is the balance between bile acids conjugation and deconjugation is important and how shifting that balance can transform the course of a disease. I’m confident this will make a difference, paving the way for new treatments, like medicines or diets, that tweak bile acids to better fight gut diseases.
How do you see these discoveries contributing to larger conversations related to organism health and future applications?
Our research shows that both host and microbially conjugated BAs may provide benefits to those with IBD, but their effectiveness hinges on a delicate balance between conjugation and deconjugation processes. This balance is largely driven by the presence of bile salt hydrolase (bsh) genes in gut bacteria. Looking ahead, our research will focus on how bsh genes regulate bile acid metabolism balance, potentially leading to targeted therapies that fine-tune this equilibrium to treat IBD.
Where might your research take you next? Are there unanswered questions the Quinn Lab might like to pursue?
There’s so much more to explore! Our future research will build on this study, focusing on the role of Ruminococcus gnavus, a key gut microbe, and its bile salt hydrolase (bsh) genes in regulating the balance of bile acid conjugation and deconjugation in IBD. We’re particularly interested in understanding how these microbial processes influence inflammation and whether targeting bacterial bsh activity could offer new ways to manage IBD.
To learn more about the Quinn Lab's work with the gut microbiome, be sure to check out these stories and more.



