Current Research
Synthetic Biology in Plant Natural Compounds
The best chemists are green. We focus on discovery of plant pathways for bioactive diterpenoids found in medicinal plant species. The functional parts allow the engineering of biotechnological chassis systems for biosustainable production.
Terpenoids (isoprenoids) are the largest and most ancient class of specialized metabolites on the planet. An amazing structural diversity reflects many biological functions that evolution has spawned. Diterpenoids, with their twenty-carbon backbone, constitute roughly a fourth of all known plant terpenoids, which currently are represented by more than 12,000 (and counting) known structures.
With only one notable exception all plants need the diterpenoid phytohormone gibberellic acid for growth and development (general metabolism). A few plant species have evolved to produce highly specialized, sometimes unique bioactive diterpenes, with important roles in plant defense. For humans, these plants are recognized since ancient times in traditional medicine, and as flavors and fragrances. More recently, diterpenoids extracted from their plant source are exploited for their analgesic, flavoring, antimicrobial, antifungal, contraceptive and psychoactive properties, and other potential industrial applications. Prominent examples of high-value plant based pharmaceuticals are the diterpenoids forskolin and ingenol-3-angelate, molecules for treatment of heart failure and skin conditions leading to cancer.

Despite the high commercial potential, industrial applications for diterpenoids are limited for a number of reasons: low amounts accumulating in the native plants, challenges with cultivation, harvest, extraction and purification of the target compound, often only a fraction of a mixture of related, but undesired compounds. In addition, chemical synthesis is currently economically not feasible due to the structural complexity.
The discovery and transfer of the biosynthetic routes from the native plant species is critical for engineering of biotechnological production hosts for biosustainable production. We have recently targeted 10 medicinal plant species accumulating structurally unique diterpenoids and discovered a dazzling rich repertoire of the biosynthetic enzymes [Zerbe, Hamberger et al., 2013]. Our aim is to establish, with those enzymes, sustainable platforms for production of plant-derived specialized metabolites, "cellular factories", with focus on bioactive pharmaceuticals or nutraceuticals with highly complex structure and stereochemistry belonging to the diterpenoid (C-20) class of compounds.
Diterpenoids are structurally highly complex, but the biosynthetic routes are relatively simple: only two enzyme families are involved in the key steps. Geranylgeranyl-diphosphate (GGPP), the ubiquitous C-20 precursor for all diterpene biosynthesis is cyclized by diterpene synthases (diTPS). The resulting diterpenes are further functionalized by enzymes of the cytochrome P450 mono-oxygenase class (P450), drivers of chemical diversification [Hamberger and Bak, 2013].
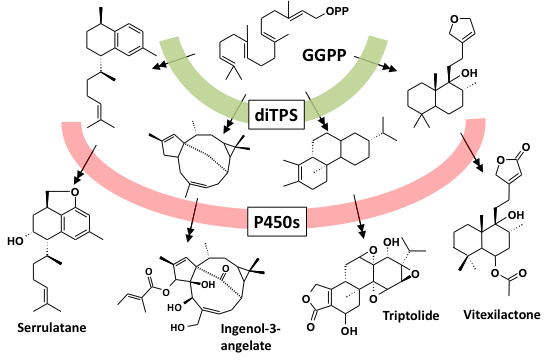
We recently discovered the first steps of the pathway to forskolin in a single cell-type of the medicinal plant Coleus forskohlii, where the high-value diterpenoids accumulate in lipid bodies [Pateraki, Andersen Ranberg et al., 2014].
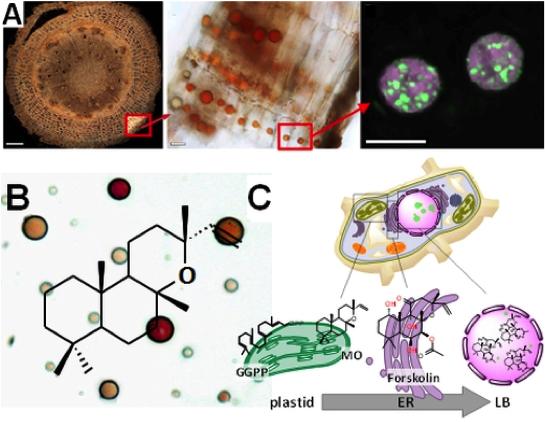
Forskolin biosynthesis in Coleus forskohlii. A. Lipid bodies are found in a cell-type of the outer root cork and Nile red staining shows a heterogeneous lipid composition in green and red. B. (13R) manoyl oxide, precursor of forskolin is detected in purified lipid bodies. C. Schematic localization of the biosynthetic route to forskolin starting in plastids, with oxidative decoration happening at the endoplasmatic reticulum and storage of diterpenes in lipid bodies.
Discovery of the functional parts from diverse plant species has opened the door to combinatorial biosynthesis: we have recently succeeded in building several neo-natural pathways by pairing enzymes in novel combinations [Andersen Ranberg et al., 2016].
Selected publications
- Recommended as a good overview on the impact of diverse P450s on the diverse plant metabolism: Hamberger B and Bak S. (2013). Plant P450s as versatile drivers for evolution of species-specific chemical diversity. Philos Trans R Soc Lond B Biol Sci.;368(1612):20120426. doi: 10.1098/rstb.2012.0426.
- Recommended as background for the use (and trouble with) P450s in engineering of pathways: Renault H, Bassard JE, Hamberger B, Werck-Reichhart D. (2014). Cytochrome P450-mediated metabolic engineering: current progress and future challenges. Curr Opin Plant Biol. 2014 Apr 5; 19C:27-34.
- A simple method description on how to transform the moss Physcomitrella patens: Spanner Bach S, King BC, Zhan H, Toft Simonsen H and Hamberger B. (2014). Heterologous stable expression of terpenoid biosynthetic genes using the moss Physcomitrella patens. Methods in Molecular Biology, Volume Plant Isoprenoids, Editor Rodiguez M. Methods Mol Biol. 2014;1153:257-71. PMID: 24777804
- Here, the heterologous transient expression in Nicotiana benthamiana is explained: Spanner Bach S, Bassard JE, Andersen?Ranberg J, Møldrup ME, Toft Simonsen H and Hamberger B. (2014). High throughput testing of candidate genes for terpenoid biosynthesis using transient expression in Nicotiana benthamiana. Methods in Molecular Biology, Volume Plant Isoprenoids, Editor Rodiguez M. Methods Mol Biol. 2014;1153:245-55. PMID: 24777804
- Recommendation as starting point in sequencing and mining of medicinal plants: Zerbe P*, Hamberger B*, Yuen M, Chiang A, Sandhu H, Madilao L, Ngyuen A, Hamberger B, Spanner Bach S and Bohlmann J. (2013). Gene discovery of modular diterpene metabolism in non?model systems. Plant Physiol. 162(2):1073-91. PMID: 23613273
- Discovery of the functional parts making diterpenes in Coleus and the discovery of lipid droplets in those cells: Pateraki I, Andersen-Ranberg J, Hamberger B, Heskes AM, Martens HJ, Zerbe P, Bach SS, Møller BL, Bohlmann J and Hamberger B (2014). Manoyl oxide (13R), the biosynthetic precursor of forskolin, is synthesized in specialized root cork cells in Coleus forskohlii. Cover story March 2014 Plant Physiol.; 164(3):1222-36. PMID: 24481136
- We recently discovered, that the functional parts can be mixed and matched to assemble ‘neo-natural’ pathways, just like Lego: Andersen-Ranberg J, Kongstad KT, Nielsen MT, Jensen NB, Pateraki I, Bach SS, Hamberger B, Zerbe P, Staerk D, Bohlmann J, Møller BL, Hamberger B. (2016) Expanding the Landscape of Diterpene Structural Diversity through Stereochemically Controlled Combinatorial Biosynthesis. Angew Chem Int Ed Engl. 2016 Jan 8. doi: 10.1002/anie.201510650. PMID: 26749264
High-level production of target diterpenoids in metabolically engineered moss

Transfer of the biosynthetic steps from the original species into the moss Physcomitrella patens holds a high potential for production of targeted diterpenoids for a number of reasons. Similar to commonly used microbes P. patens can be grown in large fermenter scale. As a photosynthetic organism, P. patens does not require sugar as carbon source for metabolite production, sunlight and atmospheric carbon dioxide are sufficient.
While P. patens lacks GA diterpenoid phytohormones, essential in all other plants for growth and development, early intermediates are produced in substantial amounts. The committed biosynthetic step in endogenous moss diterpenoid production, catalyzed by ent-kaurene synthase (PpEKS) has been identified and the encoding gene has been disrupted, resulting in viable lines without detectable diterpenoid background. These knock-out lines have an imminent capacity for production of foreign diterpenes using diTPS from other plant species as demonstrated in a pilot study using the bifunctional conifer diterpene synthase PaLAS. With this system we are approaching one of the main limitations in metabolic engineering of terpenoid pathways: functional expression of P450s, membrane bound enzymes typically located to the ER of the plant cells.