Current Research
The Hu lab has broad interest in structural biochemistry of macromolecules important in biology and biomedicine with a focus on bio-metal utilization and homeostasis. By deploying multidisciplinary approaches, including those in structural biology (x-ray crystallography, cryo-EM, and NMR), biochemistry, biophysics, and cell biology, we aim to clarify the working mechanisms of macromolecules at atomic resolution. Three major ongoing projects are outlined below.
ZIP metal transporters. The Zrt-/Irt-like protein (ZIP) family members are ubiquitously expressed in nearly all the living organisms and play a central role in homeostasis of life-essential d-block metals (primarily Zn, Fe, and Mn). We strive to clarify the structural basis of (1) the alternating access transport mechanism, (2) substrate specificity and promiscuity, and (3) substrate-dependent endocytosis of eukaryotic ZIPs. Rationally engineering plant ZIPs to reduce heavy metal (particularly Cd) contamination in food is another ongoing project. Seeking ZIP-specific inhibitors/antibodies as cancer therapeutics is also under investigation (Figure 1).
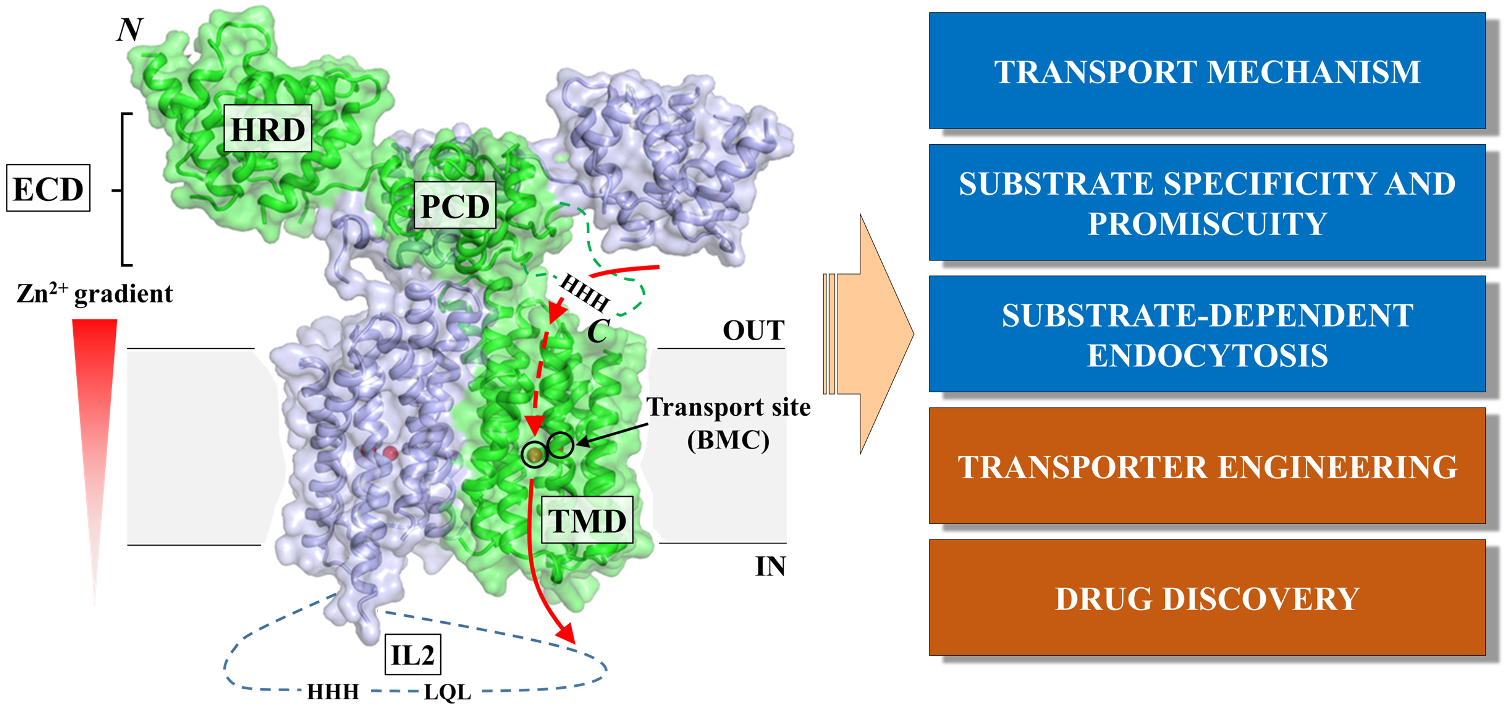
Figure 1. Overview of the ZIP project.
Lar proteins. LarA from Lactobacillus plantarum (LarALP) is the founding member of the LarA racemase/epimerase family. The activity of LarALP relies on a newly-discovered Ni-pincer nucleotide (NPN) cofactor which is biosynthesized by three novel enzymes LarB, LarC and LarE in the lar operon. We have been collaborating with Dr. Robert P. Hausinger in MMG to (1) establish the structural basis of catalysis conducted by LarALP and LarA homologs broadly distributed in prokaryotes, and (2) clarify the process and the underlying mechanism of biosynthesis of the NPN cofactor (Figure 2).
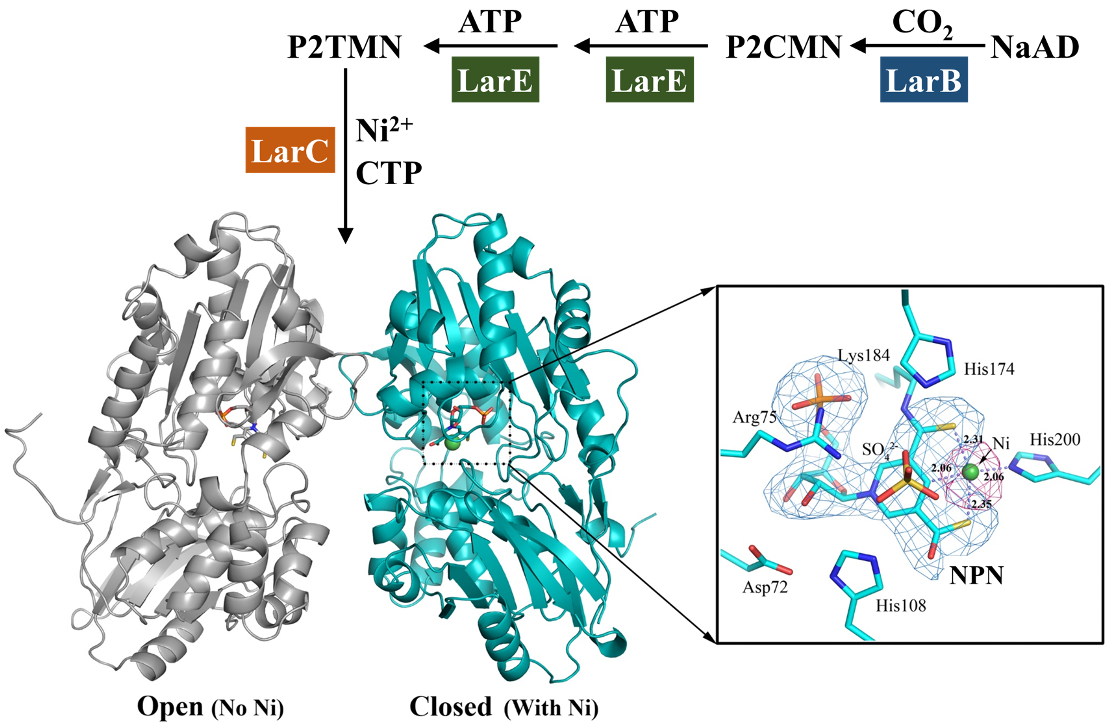
Figure 2. Biosynthesis of the NPN cofactor (upper) and the crystal structure of LarALp (lower).
PIPK lipid kinases. Phosphatidylinositol phosphate kinase (PIPK) family members produce the three types of PIP2, all of which are crucial signaling molecules involved in numerous biological processes. PIPKs are also potential drug targets for human diseases, including cancers, diabetes, inflammations, chronic pain, as well as viral infection (COVID-19 in particular). We aim to delineate the membrane sensing mechanism and the molecular basis of substrate promiscuity. We are also targeting the substrate binding site to develop non-ATP competitive inhibitors through collaboration with Dr. Xuefei Huang in Chemistry (Figure 3).
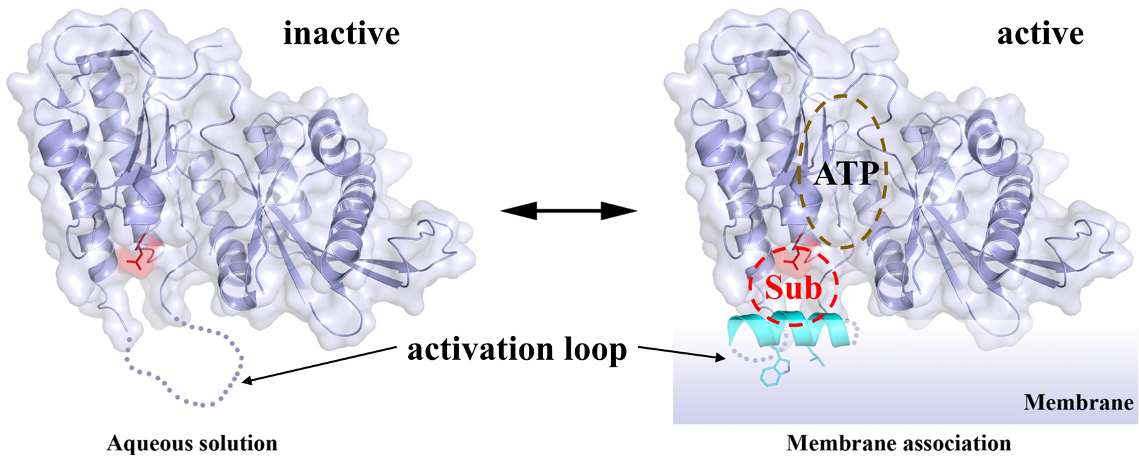
Figure 3. Activation loop (cyan) acts as a membrane sensor governing the activity of PIPKs.