Research: Project 3
Engineering lytic polysaccharide monooxygenases (LPMOs) to improve sugar digestibility
The production of biofuels from biomass requires the enzymatic breakdown of sugars from cellulose. Lytic polysaccharide monooxygenases (LPMOs), copper-containing metalloenzymes, oxidatively cleave cellulose. Because of their ability to enhance the degradation of polysaccharides, LPMOs are widely used in enzyme cocktails for biorefinery applications. While some LPMOs oxidatively cleave glycosidic bonds on the surface of only a single substrate (e.g., cellulose, chitin, and starch), other LPMOs are more promiscuous and attack multiple substrates.
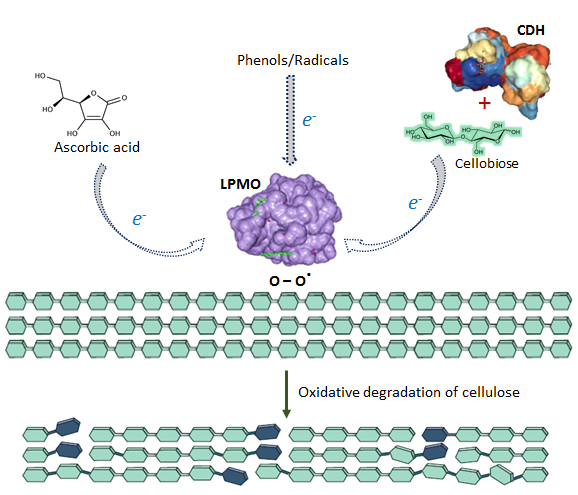
Lytic polysaccharide monooxygenases (LPMOs) act as key enzymes in the oxidative degradation of crystalline cellulose. Some of the natural electron donors are ascorbic acid, simple phenols, and cellobiose in conjunction with the enzyme cellobiose dehydrogenase (CDH).
Many biomass degrading fungi and bacteria are known to express LPMOs. Our research focuses on expressing and characterizing novel LPMOs from a variety of different organisms, with the goal of isolating enzymes amenable to industrial processes for biofuel production. In particular, we are identifying and engineering enzymes that are tolerant to residual organic solvents, stable at high temperatures, and maintain activity under a broad range of pHs.