Research: Project 6
Elucidating the biosynthesis, transport, and regulation of heme A
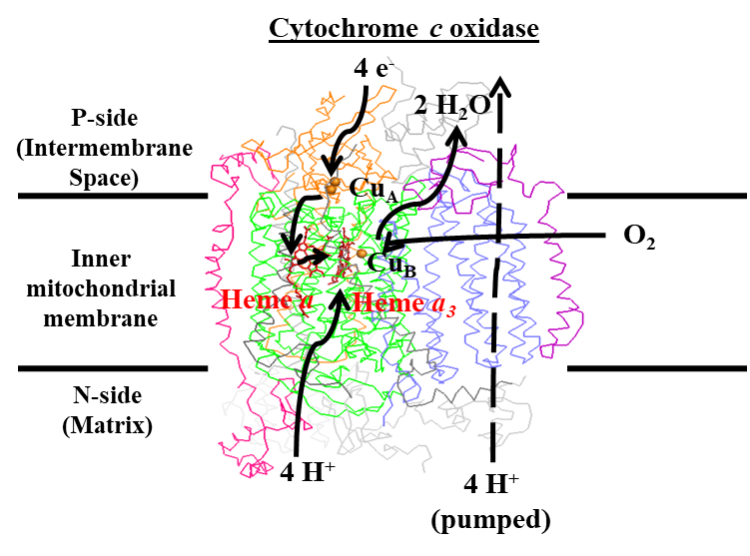 Cytochrome c oxidase (CcO) is the terminal oxidase in all plants, animals, aerobic yeasts, and many bacteria.
Arguably the most important enzyme in aerobic metabolism, CcO catalyzes the transfer of electrons from cytochrome c to O2 concomitant with the pumping of protons across the inner membrane of either mitochondria
or bacteria. This proton gradient is ultimately utilized to generate approximately
50% of the ATP formed during aerobic metabolism. The site of O2 reduction is an unusual heterobimetallic center consisting of a copper ion and a
modified heme known as heme A. The biosynthesis of heme A and its insertion into CcO is a complex process that is only just beginning to be elucidated at the molecular
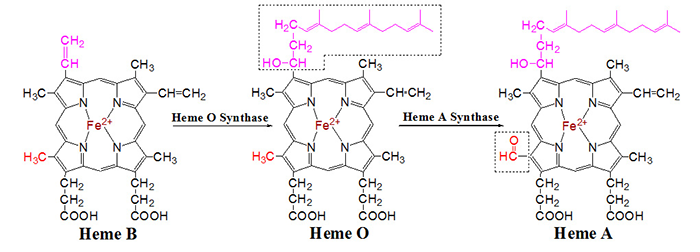
level. Heme A is derived from heme B via two enzymatic reactions. The first reaction,
catalyzed by heme O synthase, results in the conversion of the vinyl group on pyrrole
ring A into a 17-hydroxyethylfarnesyl moiety. In the second transformation, heme A
synthase utilizes a heme B cofactor to catalyze the oxidation of the methyl group
on pyrrole ring D into an aldehyde. The second reaction is especially intriguing because
this is an unusual oxidation reaction in biological systems, and the enzyme that catalyzes
this reaction is itself a heme-containing enzyme.
Cytochrome c oxidase (CcO) is the terminal oxidase in all plants, animals, aerobic yeasts, and many bacteria.
Arguably the most important enzyme in aerobic metabolism, CcO catalyzes the transfer of electrons from cytochrome c to O2 concomitant with the pumping of protons across the inner membrane of either mitochondria
or bacteria. This proton gradient is ultimately utilized to generate approximately
50% of the ATP formed during aerobic metabolism. The site of O2 reduction is an unusual heterobimetallic center consisting of a copper ion and a
modified heme known as heme A. The biosynthesis of heme A and its insertion into CcO is a complex process that is only just beginning to be elucidated at the molecular
level. Heme A is derived from heme B via two enzymatic reactions. The first reaction,
catalyzed by heme O synthase, results in the conversion of the vinyl group on pyrrole
ring A into a 17-hydroxyethylfarnesyl moiety. In the second transformation, heme A
synthase utilizes a heme B cofactor to catalyze the oxidation of the methyl group
on pyrrole ring D into an aldehyde. The second reaction is especially intriguing because
this is an unusual oxidation reaction in biological systems, and the enzyme that catalyzes
this reaction is itself a heme-containing enzyme.
The focus of our research is to elucidate the biosynthesis of heme A and its method of transport, including the formation of associated multi-component complexes. Because free heme is toxic to cells, the regulation and transport of hemes must be rigorously controlled, and our lab is providing important insight into how this occurs. To address these and related questions, we work with a number of different prokaryotic and eukaryotic organisms including E. coli, B. subtilis, S. oneidensis, R. sphaeroides, and S. cerevisiae. In doing so, we not only elucidate the biosynthetic mechanism of a key metabolic cofactor and enhance our understanding of how nature uses O2 to oxidize the very stable C-H bond, we also obtain fundamental information concerning heme transport and homeostasis.