Current Research
LIVE LONG AND HAPPILY EVER AFTER
The two major research projects of our lab are on (1) understanding how lifespan of cells can be preserved or extended by intracellular triacylglycerol, and (2) discovering the first therapeutic compound for Alzheimer’s disease. These two projects are supported by grants from the National Science foundation and the National Institute on Aging, NIH, respectively.
Lifespan regulation by intracellular triacylglycerol
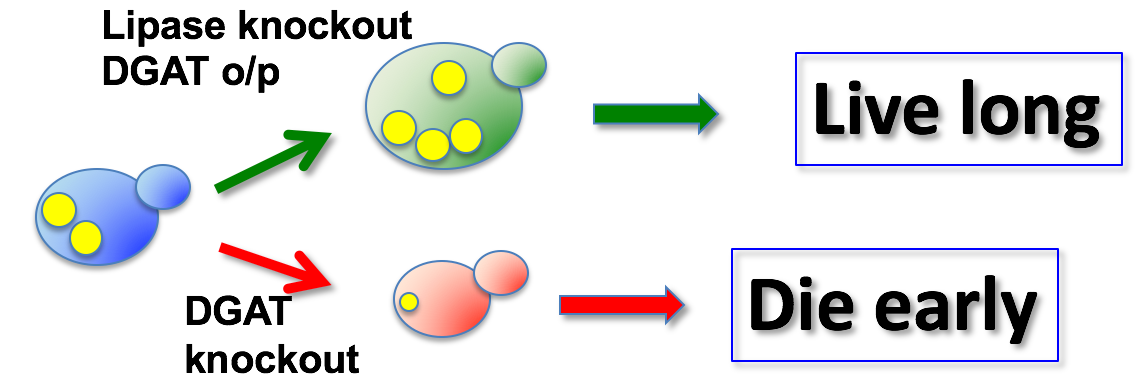 The overarching goal of this project is to uncover the molecular and mechanistic
details of lifespan regulation by intracellular triacylglycerol. Triacylglycerol (TAG;
a.k.a. triglyceride, TG) is a universal neutral lipid in eukaryotes. The best-known
function of TAG is storing excess energy. Besides energy provision, we discovered
that forcing yeast cells to accumulate TAG, by either preventing TAG hydrolysis or
raising TAG biosynthesis, extends lifespan. In contrast, blocking TAG synthesis generates
lean cells, but they die early after entering senescence. The correlation between
TAG content and lifespan suggests that intracellular TAG performs a function that
is required for long-term survival of yeast cells. This function is outside the canonical
role of energy storage, and independent of many conserved lifespan extension regimes
such as caloric restriction. We favor the hypothesis of Radicals Sink, in which intracellular
TAG stored in lipid droplets acts as a sink of metabolic free radicals that otherwise
damage biomolecules key to cell survival.
The overarching goal of this project is to uncover the molecular and mechanistic
details of lifespan regulation by intracellular triacylglycerol. Triacylglycerol (TAG;
a.k.a. triglyceride, TG) is a universal neutral lipid in eukaryotes. The best-known
function of TAG is storing excess energy. Besides energy provision, we discovered
that forcing yeast cells to accumulate TAG, by either preventing TAG hydrolysis or
raising TAG biosynthesis, extends lifespan. In contrast, blocking TAG synthesis generates
lean cells, but they die early after entering senescence. The correlation between
TAG content and lifespan suggests that intracellular TAG performs a function that
is required for long-term survival of yeast cells. This function is outside the canonical
role of energy storage, and independent of many conserved lifespan extension regimes
such as caloric restriction. We favor the hypothesis of Radicals Sink, in which intracellular
TAG stored in lipid droplets acts as a sink of metabolic free radicals that otherwise
damage biomolecules key to cell survival.
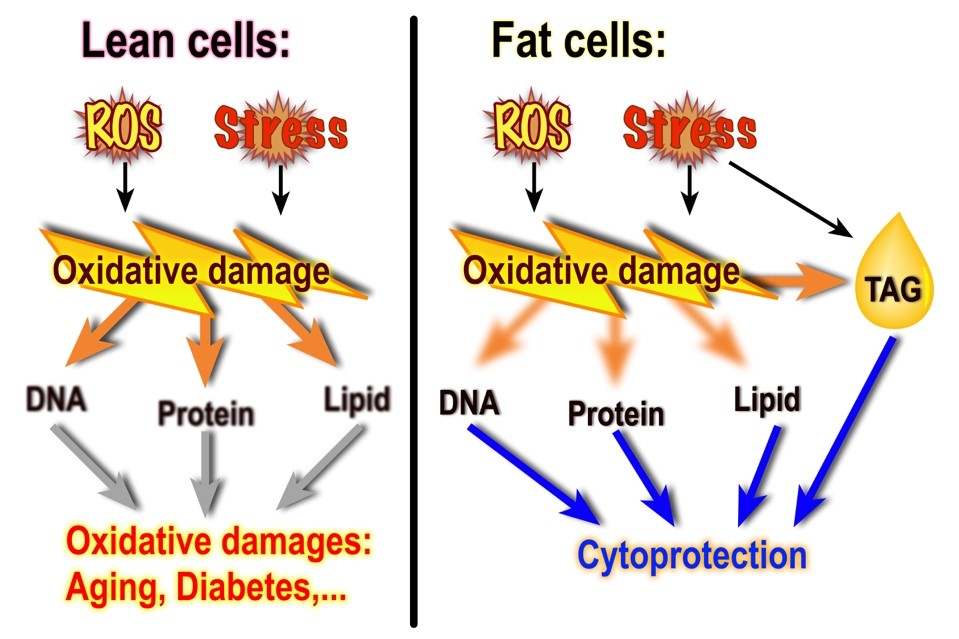 There is an increasing body of evidence supporting energy expenditure-independent
functions of neutral lipids. For example, many organisms accumulate TAG when under
stress, including starvation of a nutrient and attack of free radicals. Lipid droplets,
the organelle that stores TAG, provide anti-oxidant protection to neural stem cells
in Drosophila. In humans, a phenomenon called “obesity paradox” refers to the observations
that the lowest mortality frequently falls on the overweight (BMI between 25 and 29.5),
not the fit (BMI between 20 and 24.5) population. Similarly, “athlete’s paradox” describes
that endurance-trained athletes have higher intramyocellular lipid content while enjoying
a high oxidative capacity and enhanced insulin sensitivity. Using the unicellular
budding yeast as the model, our work will shed light on the molecular basis of longevity
and health state facilitation by intracellular triacylglycerol.
There is an increasing body of evidence supporting energy expenditure-independent
functions of neutral lipids. For example, many organisms accumulate TAG when under
stress, including starvation of a nutrient and attack of free radicals. Lipid droplets,
the organelle that stores TAG, provide anti-oxidant protection to neural stem cells
in Drosophila. In humans, a phenomenon called “obesity paradox” refers to the observations
that the lowest mortality frequently falls on the overweight (BMI between 25 and 29.5),
not the fit (BMI between 20 and 24.5) population. Similarly, “athlete’s paradox” describes
that endurance-trained athletes have higher intramyocellular lipid content while enjoying
a high oxidative capacity and enhanced insulin sensitivity. Using the unicellular
budding yeast as the model, our work will shed light on the molecular basis of longevity
and health state facilitation by intracellular triacylglycerol.
- Handee, W., Li, X., Hall, K., Deng, X., Benning, C., Williams, B., and Kuo, M.-H. (2016) An energy-independent pro-longevity function of triacylglycerol in yeast. PLoS Genetics, 12:e1005878. doi: 0.1371/journal.pgen.1005878
- Li, X., Handee, W., and Kuo, M.-H. (2017) The slim, the fat, and the obese: guess who lives the longest? Curr. Genet. 61:43-49. doi: 10.1007/s00294-016-0617-z
Alzheimer’s disease drug discovery
Alzheimer’s disease (AD) is an irreversible neurodegenerative disease affecting 47 million people worldwide, and costs 604 billion US dollars every year for medical expenses. To date, there is no cure or prevention for AD. The two defining features of AD are Ab plaques and neurofibrillary tangles that are composed of hyperphosphorylated tau. While the AD drug discovery landscape has been dominated by anti-Ab measures, recurring failures of clinical trials argue strongly that a realignment of the drug target and strategies is needed to make a breakthrough in AD therapeutics development. Indeed, multiple lines of evidence suggest that the pre-tangle stage of hyperphosphorylated tau aggregates cause diffusible cytotoxicity that likely underlies neurodegeneration. Screening for compounds that prevent hyperphosphorylated tau from forming the cytotoxic aggregates thus affords a more viable route for AD drug discovery.
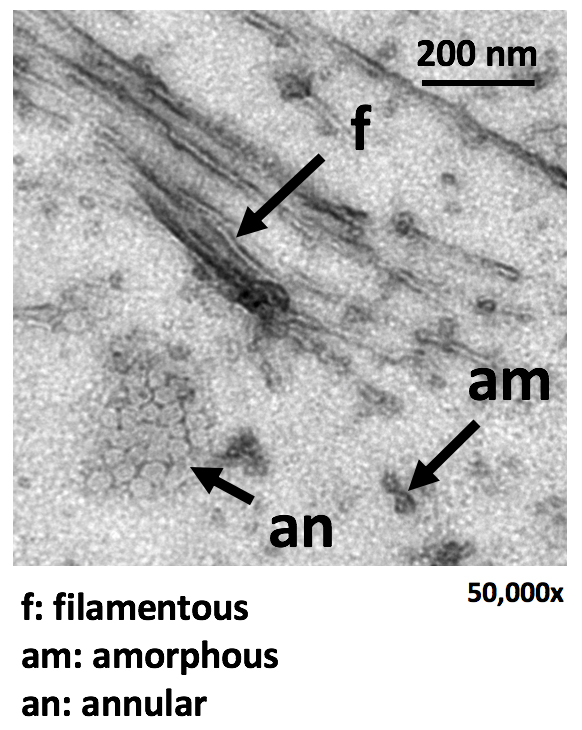 The tangle-centric drug design has yet to come to fruition, due largely to the lack
of a feasible means of preparing a tau protein bearing the pathological mark of hyperphosphorylation.
To overcome this hurdle, we have developed the PIMAX technology that produces hyperphosphorylated
tau (p-tau) in E. coli. P-tau fibrillizes autonomously (without an inducer) via morphologically
diverse intermediates (see TEM micrograph), and causes apoptosis of different cells
including a neuroblastoma cell line. This is the first hyperphosphorylated tau-based
cell model for neurodegeneration.
The tangle-centric drug design has yet to come to fruition, due largely to the lack
of a feasible means of preparing a tau protein bearing the pathological mark of hyperphosphorylation.
To overcome this hurdle, we have developed the PIMAX technology that produces hyperphosphorylated
tau (p-tau) in E. coli. P-tau fibrillizes autonomously (without an inducer) via morphologically
diverse intermediates (see TEM micrograph), and causes apoptosis of different cells
including a neuroblastoma cell line. This is the first hyperphosphorylated tau-based
cell model for neurodegeneration.
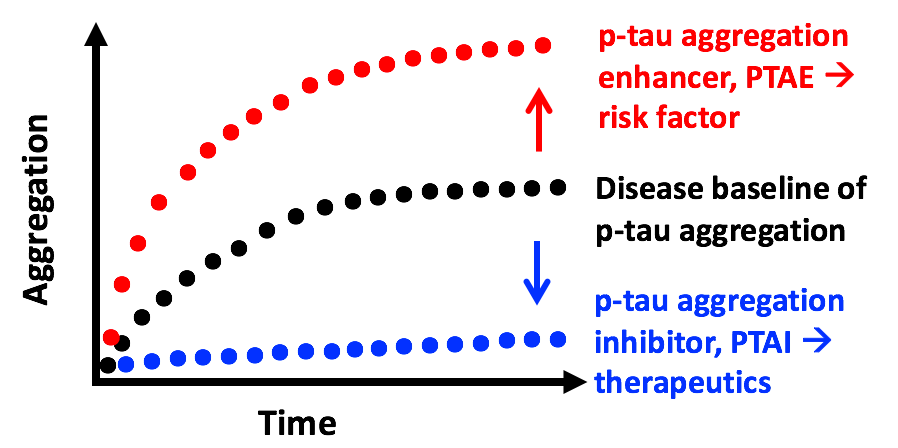 Besides using p-tau for disease mechanistic studies, we are engaged in AD drug discovery
endeavors. Our working hypothesis is that small-molecule compounds that suppress the
fibrillization of p-tau (p-tau aggregation inhibitors, PTAIs) are candidates for AD
therapeutics. In contrast, those that enhance p-tau aggregation (PTAEs) are potential
risk factors for AD and similar neurodegenerative disorders collectively known as
tauopathies. Our pilot screen of a 1280-compound library results in encouraging hits.
Lead optimization of these candidate is underway. We are currently supported by a
grant from the National Institute on Again, NIH, to conduct a 100,000-compound library
screen for novel compounds as potential AD therapeutics.
Besides using p-tau for disease mechanistic studies, we are engaged in AD drug discovery
endeavors. Our working hypothesis is that small-molecule compounds that suppress the
fibrillization of p-tau (p-tau aggregation inhibitors, PTAIs) are candidates for AD
therapeutics. In contrast, those that enhance p-tau aggregation (PTAEs) are potential
risk factors for AD and similar neurodegenerative disorders collectively known as
tauopathies. Our pilot screen of a 1280-compound library results in encouraging hits.
Lead optimization of these candidate is underway. We are currently supported by a
grant from the National Institute on Again, NIH, to conduct a 100,000-compound library
screen for novel compounds as potential AD therapeutics.
In addition to drug discovery, we collaborate with Cayman Chemical Inc. (Ann Arbor) to develop a kit that features the aggregation of p-tau in vitro. This kit will allow researchers to test their favorite compounds and biological molecules for effects on p-tau aggregation and, if positive, the potential of AD modulation by such molecules. This project has been supported by a phase I STTR (small business technology transfer) grant. The development of a phase II project is ongoing.
- a. Liu et al, Small-molecule modulators of the aggregation of hyperphosphorylated tau include potential risk factors and therapeutics for Alzheimer’s disease. J. Bio. Chem., in revision.
- Sui, D., Xu, X., Ye, X., Liu, M., Mianecki, M., Rattanasinchai, C., Buehl, C., Deng, X., and Kuo, M.H. (2015) PIMAX approach to producing challenging proteins including hyperphosphorylated tau and active CDK/p25 kinase complex. Mol. Cell. Proteomics. 14:251-62. doi: 10.1074/mcp.O114.044412
- Sui, D., Liu, M., and Kuo, M.-H. In vitro aggregation assays using hyperphosphorylated tau protein. (2015) J Vis Exp Jan 2; (95). doi: 10.3791/51537