Research: Project 1
Chromatin dynamics and regulation of nuclear activities
Chromatin is a dynamic structure that not only organizes the genome, but also contributes significantly to the regulation and execution of a variety of nuclear activities, including cell cycle control and transcriptional regulation.
 A. Histone H3 and mitotic progression and checkpoint control.
A. Histone H3 and mitotic progression and checkpoint control.
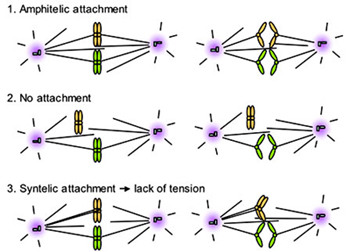
Faithful segregation of the duplicated genomes requires that both sister kinetochores are attached to mitotic spindles emanating from opposite spindle poles. Bipolar attachment results in physical tension between sister chromatids.
Cells monitor the status of tension as a means to detect any mistake in kinetochore attachment. Uncorrected attachment errors may lead to missegregation and aneuploidy, a condition frequently linked to cancer. If missegregation occurs during meiosis, trisomy results, causing miscarriage and certain congenital diseases such as Down's syndromes. Spindle assembly checkpoint is the major safety control employed by all eukaryotes to ensure bipolar attachment. Our main research goals are to understand whether and how chromatin plays regulatory and/or structural roles in facilitating mitotic fidelity and progression. Currently, we focus on one histone H3 mutation, which impairs cellular ability to detect defects in sister chromatid tension and to activate the spindle assembly checkpoint when such tension sensing defects occur. Genetic and biochemical approaches are employed for functional and structural studies.
B. Functions of histone H3 phosphorylation.
Histone H3 phosphorylation is conserved through evolution and has been used widely as a marker for mitosis progression. However, molecular functions exerted by the phosphorylated H3 remain elusive. Multiple residues within the amino terminal tail domain of H3 can be phosphorylated, singly or in different combination. We use genetic approach to dissect the functions of these different H3 modification events.